Body Centered Cubic Unit Cell. Click the links on the right to illustrate.
Unit Cell Body Centered Cubic Crystal Lattice Structures Physical E Crystal Lattice Structure Unit Cell Nomenclature Chemistry
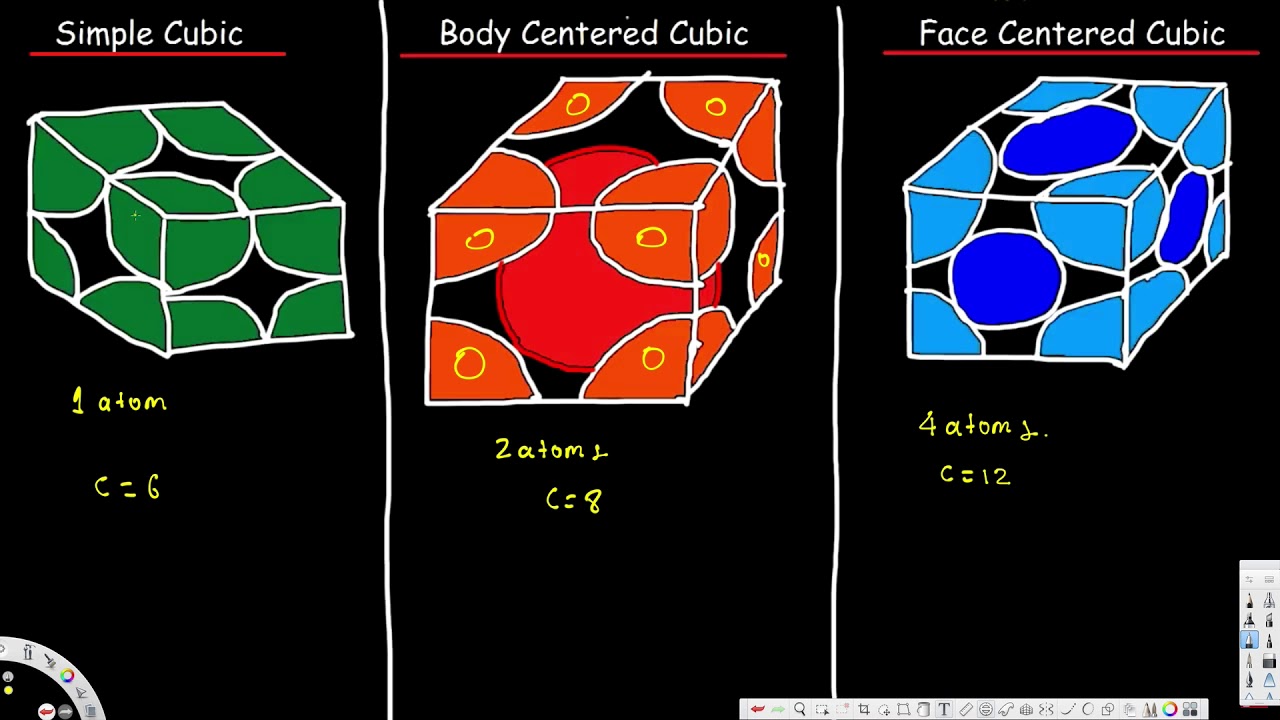
At the cells centre two atoms are present.
. The unit cell completely describes the structure of the solid which can be regarded as an almost endless repetition of the unit cell. The Body-Centered Cubic BCC unit cell can be imagined as a cube with an atom on each corner and an atom in the cubes center. An example of simple cubic unit cell with atoms at locations other than corners is CsCl.
The simple cubic unit cell is delineated by eight lattice points which mark the actual cube. Simple Cubic unit cell. BCC has 2 atoms per unit cell lattice constant a 4R3 Coordination number CN 8 and Atomic Packing Factor APF 68.
In this type of unit cell. A unit cell is the simplest repeating unit of a crystalline solid. By convention the edge of a unit cell always connects equivalent points.
The unit cell commonly selected for a simple cubic metal is a cube because this is the simplest repeating part of the lattice. If the atomic mass is known the mass of the unit cell is equal to atomic mass Avogadro number atomic mass 6022 1023g. There are 8 eighths one in each corner for a total of ONE atom in the unit cell.
15 rows The two-dimensional arrangement of the spheres can be extended to the third dimension to form a. Each corner of the unit cell is defined by a lattice point at which an atom ion or molecule can be found in the crystal. Simple Cubic Unit Cell.
This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures. The Mass of Atom is the mass of single atom present in the unit cell Volume of Unit Cell is equal to the cubed cell-edge length a. The total of atoms located in the End-Centred Cubic Unit Cell 1 1 2 atoms.
Arrangements duplicate themselves every other layer. It highlights the key differences between the sim. Each layer is offset from the layer before.
Perhaps the only elemental example is Po where Po atoms are located only at the cube corners. This unit cell uses nine atoms eight of which are corner atoms forming the cube and one more in the center of the cube. It turns out that only the metal Polonium Po has this crystal structure.
The volume of the unit cell is 8r3. This unit cell uses nine lattice points eight of which are corner atoms forming the cube. These are corner atoms so each one only contributes one eighth of an atom to the unit cell thus giving us only one net atom.
The simple cubic unit cell is a cube all sides of the same length and all face perpendicular to each other with an atom at each corner of the unit cell. The corners contribute only one net atom and. The Density of Simple Cubic Unit Cell formula is defined as the ratio of mass of all atoms to the volume of unit cell and is represented as ρ M V unit cell Avaga-no or Density Mass of Atom Volume of Unit Cell Avaga-no.
They vary in how the atomsspheres are arranged inside of it. Given below is the table representing the total number of. A simple cubic unit cell contains one atom.
What is Simple Cubic Unit Cell. The edges of the unit cells are of equal length and the cell angles are 90 degrees. The coordination number is 6 there is 1 atom per cell and the length of.
19 rows Simple Cubic Unit Cell is the least complex cubic unit cell and contains only 1 atom. The unit cell completely describes the structure of the solid which can be regarded as an almost endless repetition of the unit cell. Canvas is matched to your browser window.
This structure is the simple cubic crystal structure. Then put the first square on the second square to form a cube with eight atoms one at each corner. The simple cubic unit cell is delineated by eight atoms which mark the actual cube.
The simplest unit cell is the primitive cubic unit cell in which there are atoms at each of the 8 corners of a cube. Start by taking four atoms and arranging them in a square. It is one of the most common structures for metals.
The volume of the unit cell is readily calculated from its shape and dimensions. The volume of the unit cell is readily calculated from its shape and. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure.
Use your fingers or mouse to control the model hold shift key or use mouse wheel to zoom it. The simple cubic unit cell is a cube all sides of the same length and all face perpendicular to each other with an atom at each corner of the unit cell. Each atom is making a contribution of 12th cells portionpart.
Then take four more atoms and arrange them in a square. Simple cubic unit cell is a cubic unit cell with lattice points not atoms only at the corners. They are called simple cubic face-centred cubic and body-centred cubic.
Additional information on the unit cell is provided on the next page. Crystal models updated 15 Oct 2011. Hence density of unit cell mass of unit cell volume of unit cell.
These are corner atoms so each one only contributes one eighth of an atom to the unit cell thus giving us only one net atom.
Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell The Unit Atom
Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell Nomenclature Chemistry Chemistry
Face Centered Cubic Fcc Unit Cell Earrings For Materials Scientists Unit Cell Materials Scientist Bravais Lattice
Chemistry Liquids And Solids 32 Of 59 Crystal Structure Seven Types Of Unit Cells Crystal Structure Chemistry Unit Cell
11 7 Structure Of Solids Chemistry Libretexts Unit Cell Material Science Physical Chemistry
Body Centered Cubic Model In Silver Interestingly The Bcc Unit Cell Was The Inspiration For The Atomium In Belgium But The Unit Cell Science Decor Crystals
Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell Chemistry The Unit
Unit Cell Simple Cubic Body Centered Cubic Face Centered Cubic Cryst Unit Cell Crystal Lattice Structure Nomenclature Chemistry
Types Of Unit Cell Unit Cell The Unit Cell
Unit Cell Simple Cubic Structure Physical Electronics In 2021 Unit Cell Physics The Unit
Primitive And Non Primitive Unit Cell In 2022 Unit Cell The Unit Primitive
Basic Crystal Concepts Bravais Lattice Unit Cell Crystal Lattice
Unit Cell Face Centered Cubic Crystal Lattice Structures Crystal Lattice Structure Unit Cell Nomenclature Chemistry
Planar Packing Fraction Factor For The Body Centred Cubic 111 Plane Fractions Body Packing
Pin By Flathorn On Sacred Geometry Bravais Lattice Chemistry Chemistry Education
Face Centered Cubic Fcc A Closest Packing Structure Lattice Structure Crystalline Solid Chemistry
Cubic Lattice From Wolfram Mathworld Cell Forms Unit Cell Lattice
- panau di muka
- braces kerajaan untuk pelajar 2020
- contoh surat tunjuk sebab tidak hadir
- harga bateri kereta nissan
- ikan badan putih masak cuka
- contoh kad kahwin sekeping
- 16 mei 2018 kalendar islm
- kelopak mata kanan atas
- ahli iz one nama
- homestay taman aman anak bukit
- malaysian society?"
- one pint normal saline
- muka merah merah cendol
- taman bayu puteri johor
- ecosky taman wahyu price
- gambar animasi warna kelabu
- cara masak ayam masak merah utara
- rock road seafood menu
- undefined
- simple cubic unit cell
